Weight-loss drug effective in treating apnea, study finds
June 21 (UPI) -- Treating obstructive sleep apnea with a type 2 diabetes medication may offer new hope for sufferers of the sleep disorder, a new study released Friday indicates.
Researchers at University of California-San Diego School of Medicine and international collaborators ran a year-long study in which 469 sleep apnea sufferers in the United States and six other countries were either given 10 or 15 milligrams of tirzepatide, which is approved for weight loss, or a placebo, UC said in a news release.
The study was double-blind, meaning neither the patients nor the investigators knew who was receiving the actual medication.
The results, published in the New England Journal of Medicine, found that tirzepatide led to a significant decrease in the number of breathing interruptions during sleep, a key indicator used to measure the severity of sleep apnea -- far greater than what was seen in the control group.
"This study marks a significant milestone in the treatment of OSA, offering a promising new therapeutic option that addresses both respiratory and metabolic complications," said Dr. Atul Malhotra, lead author and professor of medicine at University of California San Diego School of Medicine and director of sleep medicine at UC San Diego Health.
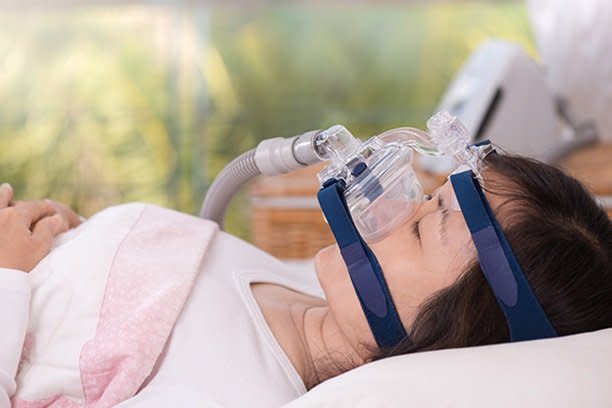
Study participants included both patients receiving continuous positive airway pressure therapy -- in which a machine keeps the airway open during sleep to prevent interruptions in breathing -- and those who were not, but all had a clinical obesity diagnosis.
Significantly, those on tirzepatide -- the active ingredient in Zepbound -- improved to the point where CPAP might not be necessary, the researchers said, noting that existing data strongly indicated drug therapy that targets both sleep apnea and obesity delivers greater benefits rather than treating either condition separately.
The most common side effect reported was mild stomach issues.
The study was in part funded by tirzepatide developer, Eli Lilly, and co-authored by four of the U.S. pharma giant's medical executives.
The reduced oxygen levels in the blood caused by the repeated episodes of irregular breathing due to complete or partial blockage of the upper airway that characterize OSA are also linked to increased risk of hypertension and heart disease, with earlier studies by Malhotra estimating as many as 936 million sufferers worldwide.
The study claimed tirzepatide also cut the risk factors for these related cardiovascular diseases and improved body weight.
Malhotra hailed the new approach as a breakthrough in the management of obstructive sleep disorder, with the potential to improve the quality of life for many of those affected by the condition for whom previously the only option was intrusive devices such CPAP, but that only work when used consistently.
"This new drug treatment offers a more accessible alternative for individuals who cannot tolerate or adhere to existing therapies. We believe that the combination of CPAP therapy with weight loss will be optimal for improving cardiometabolic risk and symptoms," Malhorta said.
"Tirzepatide can also target specific underlying mechanisms of sleep apnea, potentially leading to more personalized and effective treatment. It means we can offer an innovative solution, signifying hope and a new standard of care to provide relief to countless individuals and their families who have struggled with the limitations of existing treatments.
The team plan to follow-up the research by conducting clinical trials to investigate the effects of tirzepatide when used over the longer term.
Co-authors of the study also came from University of Sydney; University Hospital Berlin; University of Illinois Chicago; Harvard Medical School; and the University of Pennsylvania.
